China conditionally approves four self-developed COVID-19 vaccines: official
Share - WeChat

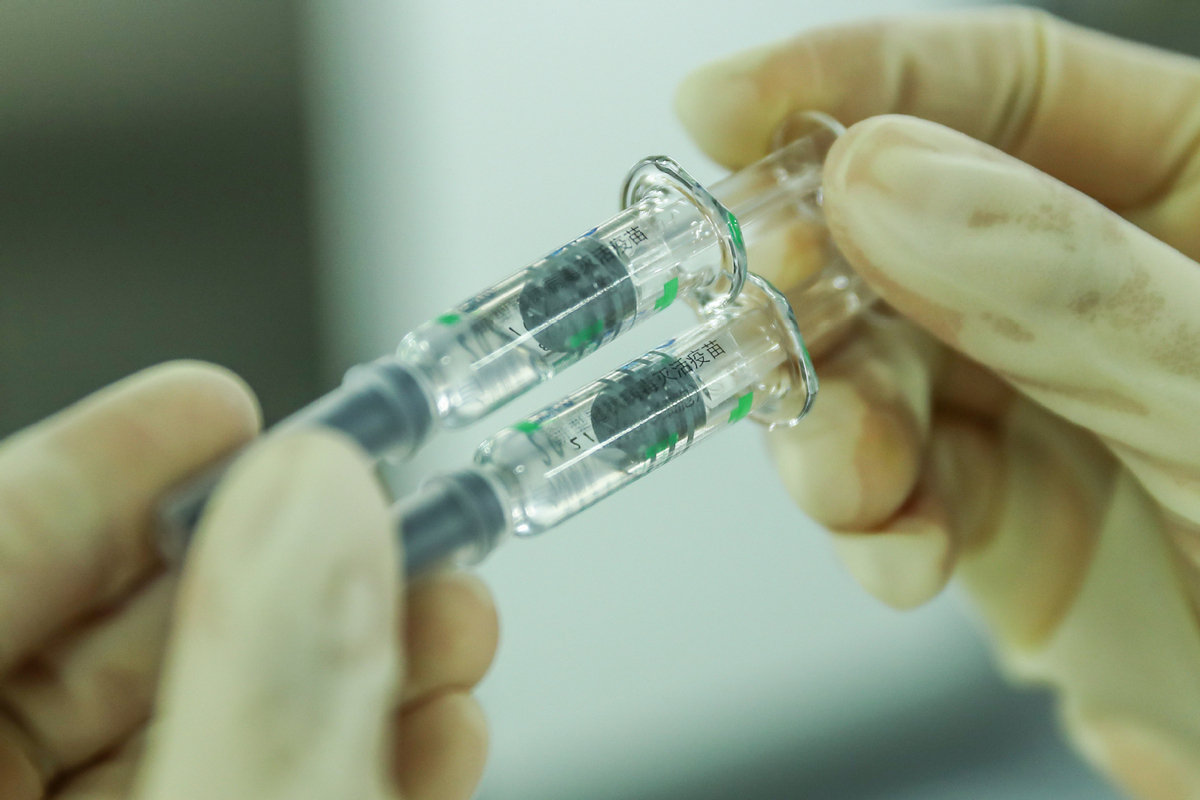
BEIJING - China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.
- Zhangjiajie International Acrobatic Festival showcases cultural exchanges
- China's role in advancing gender equality through education highlighted
- Top court nurtures growth of seed sector
- Dalian patent fair boosts innovation drive
- Weifang charts robust foreign trade growth, sets sights on further opening-up
- Full text of Chinese President Xi Jinping's keynote address at opening ceremony of the Global Leaders' Meeting on Women